ADMA Biologics (NASDAQ: ADMA) has
emerged as a standout performer in the healthcare sector, demonstrating robust
financial growth and operational efficiency. Following its Q1 2025 earnings
report, the company continues to showcase its potential as a compelling
investment opportunity, driven by strong revenue growth, expanding profit
margins, and strategic initiatives.
1. About ADMA Biologics
Founded in 2004 and headquartered
in Ramsey, New Jersey, ADMA Biologics specializes in developing, manufacturing,
and marketing plasma-derived biologics for treating immune deficiencies and
infectious diseases. The company's product portfolio includes BIVIGAM, an
intravenous immune globulin (IVIG) for primary humoral immunodeficiency;
ASCENIV, an IVIG product for primary immunodeficiency; and Nabi-HB, a human
polyclonal antibody for hepatitis B exposure. ADMA operates plasma collection
facilities under the ADMA BioCenters brand, ensuring a vertically integrated
supply chain.
2. ADMA Biologics Financial
Performance
ADMA Biologics reported another
quarter of strong financial results in Q1 2025, reinforcing its position as a
high-growth and profitable player in the biotech space. The company posted
quarterly revenue of $114.8 million, up 40.22% from $81.88 million in Q1 2024.
This growth reflects increased product demand, operational efficiencies, and
the continued ramp-up of its intravenous immunoglobulin (IVIG) products,
particularly ASCENIV and BIVIGAM. Earnings per share (EPS) for the quarter came
in at $0.11, a 41.39% increase over $0.08 from the same period last year,
highlighting improved profitability and scale.
On a trailing twelve-month (TTM)
basis, revenue grew significantly to $459.38 million, a 62.22% increase from
$283.18 million in the prior year. The company also posted TTM EPS of $0.88,
marking a turnaround from the previous year's loss of $0.02, indicating a shift
to sustained profitability. Free cash flow per share (TTM) also saw a
substantial rise, reaching $0.37 compared to $0.07 a year earlier, an
impressive 428.57% increase. This improvement in free cash flow demonstrates
ADMA’s ability to convert earnings into cash efficiently, a critical metric for
long-term sustainability.
ADMA's profitability metrics
remain exceptionally strong. The company achieved a gross profit margin of
52.58%, a net profit margin of 45.01%, and a free cash flow margin of 19.73%,
showcasing both operational efficiency and solid cost management. Return on
assets stood at a healthy 22.06%, while return on equity soared to 78.45%,
reflecting the company’s ability to generate high returns for shareholders with
relatively modest leverage. In fact, ADMA maintains a low debt-to-equity ratio
of 0.22, indicating a conservative capital structure that adds a layer of financial
stability.
Over the past five years, ADMA
Biologics has grown its revenue at a 75.4% CAGR, while net income and free cash
flow turned positive in 2024 and now have strong margins.
3. ADMA Biologics 2025 Financial
Forecast
Looking ahead, analysts project continued top-line growth, forecasting full-year 2025 revenue to reach $511.3 million, a 19.9% increase from 2024. While EPS is expected to decrease to $0.63 from $0.85 in 2024, representing a 21.91% decline, this is because there was provision for income tax in 2024 which could be excluded in 2025. Despite this temporary dip in EPS, the long-term outlook remains overwhelmingly positive. Analysts have set a 12-month price target of $30.67 on the stock with Strong Buy rating, implying a potential upside of 59.74% from current levels.
4. ADMA Stock Price Performance
and Valuation
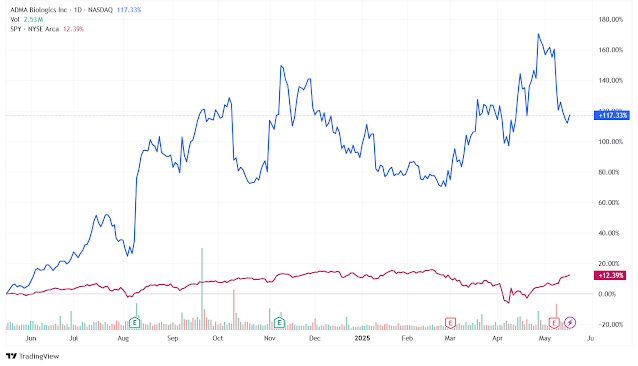
At the time this article is
written ADMA Biologics stock is trading at $19.69. Over the past year, the
stock has delivered an impressive return, surging 117.3% significantly outperformed
the broader S&P 500 index, which rose only 12.3% during the same period.
Looking further back, the stock has appreciated a remarkable 593.31% over the
last five years, again outperforming the S&P 500's 101.1% gain over that
span. Such strong price momentum reflects growing investor confidence in the
company’s execution, profitability, and long-term growth potential.
From a valuation standpoint, ADMA
still offers an attractive investment opportunity despite its significant price
appreciation. The stock trades at a trailing twelve-month (TTM) price-to-sales
(P/S) ratio of 10.09 and a forward P/S of 9.19, which, while elevated, are
supported by the company’s high growth rate and expanding margins. Its trailing
P/E ratio stands at 23.56, and the forward P/E is 31.13. Additionally, the
price-to-free cash flow (P/FCF) ratio of 51.86.
Based on data from Finchat, if we
look at the valuation since 2024, ADMA Biologics has been trading slightly
above its one-year average valuation. Its forward P/FCF is below the average of
35.15, while its forward P/E is above the average of 23.38, and its forward P/S
is above the average of 7. ADMA Biologics is still experiencing strong growth,
so these valuations may still be reasonable.
5. ADMA Biologics Growth
Potential
ADMA's growth prospects are
underpinned by several factors.
- Strategic Growth Catalysts and
Manufacturing Innovation
ADMA Biologics’ growth is propelled by the FDA’s April 2025 approval of its innovative yield enhancement process, increasing production yields by about 20% from the same plasma volume for ASCENIV and BIVIGAM. As the first U.S. plasma-derived product maker to gain such approval, ADMA gains a significant manufacturing efficiency advantage.
This breakthrough is expected to boost revenue and earnings starting late 2025 and accelerate through 2026 by improving supply chain economics and expanding margins. Additionally, ADMA continues investing in R&D, advancing its pipeline with programs like SG-001, a preclinical hyperimmune globulin targeting Streptococcus pneumoniae. These initiatives position ADMA to diversify its product portfolio and strengthen its market presence in plasma therapeutics. - Capital Allocation and
Financial Strength
ADMA's growth strategy is complemented by prudent capital allocation decisions that balance reinvestment with shareholder returns. The company recently authorized a substantial $500 million share repurchase program, representing approximately 8% of its current market capitalization. This buyback authorization signals management's confidence in the company's valuation and future prospects while potentially enhancing earnings per share through reduced share count.
Additionally, ADMA has strengthened its financial position through strategic debt reorganization, which reduced its cost of debt capital by 1.1%. This improvement in capital structure provides greater financial flexibility and reduces interest expense, potentially accelerating the pace of earnings growth in future periods. - Updated Financial Guidance and
Long-Term Outlook
Following the strong Q1 performance and FDA approval of the yield enhancement process, ADMA has increased its financial guidance for the coming years. The company now projects total revenues exceeding $500 million for fiscal year 2025 and more than $625 million for 2026. These revised projections represent substantial increases from previous guidance and reflect management's growing confidence in the business trajectory.
Even more impressive is ADMA's long-term revenue expectation of achieving $1.1 billion in annual revenue before 2030. This ambitious target would represent nearly a tenfold increase from the company's 2023 annual revenue, signaling management's bold vision for continued expansion.
6. Risks to Consider
While ADMA presents a compelling
investment case, potential risks include.
- Product Supply Constraints
A particularly concerning risk involves ADMA's specialized raw material requirements. The company collects and processes plasma containing high-titer antibodies to RSV to manufacture ASCENIV using their patented proprietary microneutralization assay. However, the high-titer plasma meeting their internal specifications represents less than 10% of total donor collection samples tested. This creates a significant supply constraint that could limit production capacity and growth potential. - Commercialization vs. R&D
Balance
Since receiving FDA approval for ASCENIV, ADMA has been working to commercialize the product while simultaneously continuing research and development activities. The company has acknowledged that there can be no assurance they will successfully manage this balance. This dual focus creates potential risks for investors, as the company must allocate resources between current product marketing and future pipeline development. - Product Pipeline Development
The development of new product candidates requires significant additional research, clinical trials, and regulatory approvals. ADMA must overcome substantial regulatory hurdles before commercializing new products in the United States and other countries. Furthermore, expanding commercial operations requires significant funding, with no guarantee that after substantial expenditure, the company will successfully develop, commercialize, or generate significant revenues from these products.
Conclusion
ADMA Biologics has demonstrated
remarkable financial performance in Q1 2025, with substantial revenue and
profit growth, strong margins, and a solid balance sheet. The company's
strategic initiatives, including product expansion and manufacturing
enhancements, position it for continued success in the growing IVIG market. Despite
potential risks, ADMA's robust fundamentals and growth prospects make it a
compelling investment opportunity for those seeking exposure to the biotech industry.




Comments
Post a Comment